Abstract
Introduction Despite the use of novel induction regimens, stem cell transplantation (SCT), and maintenance therapy, plasma cell leukemia (PCL) remains a challenging disease with a dismal prognosis. PCL occurring de novo is referred to as primary PCL (pPCL) while secondary PCL (sPCL) refers to disease progressing from antecedent multiple myeloma and is typically associated with inferior outcomes. Retrospective studies have largely focused on pPCL, leaving little to be known about sPCL. Currently, there is no agreed standard of care management for PCL and most approaches are based on small prospective, retrospective studies and institutional experience. We conducted a multicenter retrospective analysis of the clinical presentation, treatment, and outcomes of 130 patients with PCL.
Methods Data of patients diagnosed with pPCL or sPCL between January 2010 and January 2021 were entered into a study-specific REDCap database from 7 different U.S. academic sites. PCL was defined as ≥ 5% circulating plasma cells according to the 2021 IMWG consensus definition. Clinical data included baseline patient characteristics, clinical presentation, treatment, therapeutic response, and survival outcomes. Overall survival (OS) curves were plotted using the Kaplan-Meier method. Cox proportional hazards regression tested associations between patient characteristics and OS, generating hazard ratios (HR) and 95% confidence intervals (CI), adjusted for PCL type (primary or secondary) and SCT.
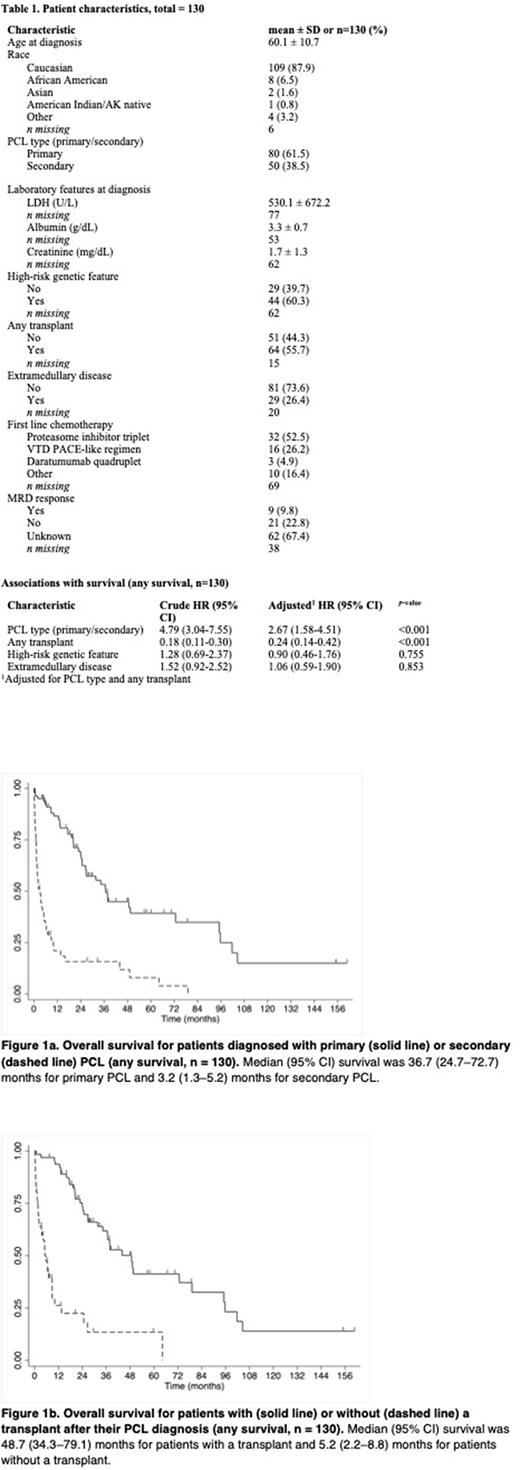
Results The analytical cohort included 80 pPCL and 50 sPCL patients. Median age at diagnosis was 60 years. High-risk cytogenetics were found in 60.3% of the patients where it was documented. Of the 60 patients with a documented induction regimen, 53.3% received a proteasome inhibitor triplet, 26.7% received a VTD-PACE like conventional chemotherapy, and 5% received a daratumumab quadruplet regimen. Extramedullary disease was documented in 26.4% of the patients and SCT (either autologous or allogeneic) was done in 55.7% of the entire cohort (table 1). The median OS for all patients, those with pPCL and those with sPCL was 22.8, 36.7, and 3.2 months, respectively. Secondary PCL was associated with worse survival outcomes compared with pPCL (HR, 2.67; 95% CI, 1.58-4.51; p<0.001) (table 1, figure 1a). Median OS was better in patients treated with a proteasome inhibitor triplet regimen vs VTD PACE-like combination (36.6 versus 13.1 months) although this variable was missing in over half of the cohort. OS was prolonged among patients who underwent any type of SCT compared with those who did not undergo SCT (48.7 versus 5.2 months, p<0.001) (figure 1b). No significant association between SCT type and OS was observed. Amongst the entire cohort, sPCL and extramedullary disease were independently associated with poor outcomes.
Conclusion This multicenter retrospective study is one of the largest PCL analyses performed to date and reveals the clinical practice patterns of treatment and survival of PCL patients across the U.S. in the novel treatment era. The survival analysis reinforces the poor prognosis in sPCL patients and the continued need for novel treatment approaches in this patient population. While limited by retrospective design, this analysis suggests prolonged survival with transplantation in both pPCL and sPCL.
Disclosures
Sborov:Sanofi: Consultancy; Abbvie: Consultancy; Amgen: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Bioline: Consultancy; BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Green:Neoleukin Theraeutics: Membership on an entity's Board of Directors or advisory committees; Janssen Biotech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Legend Biotech: Consultancy; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; SpringWorks Therapeutics: Research Funding; Juno Therapeutics: Patents & Royalties, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Cellectar Biosciences: Research Funding. Liedtke:Gilead: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Natera: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Caelum: Research Funding; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Membership on an entity's Board of Directors or advisory committees; Allogene: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Nadeem:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GPCR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal